
Welcome to
Zari Chemistry .
This is group 15
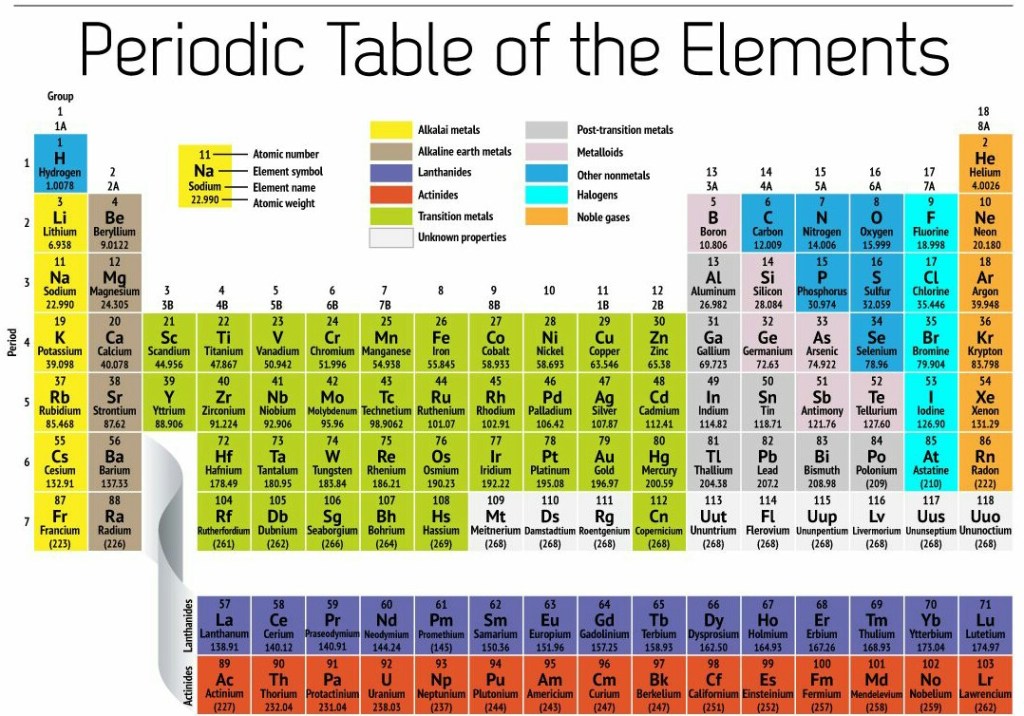
Group 15 elements
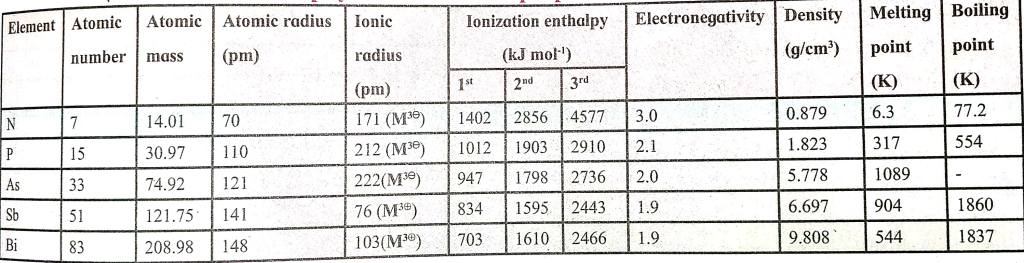
Nitrogen

electronic configuration 1s22s22p3.
Nature ----> Acidic
Non metal

Electronegativity 3.0
Oxidation states -3 to +5




Phosphorus

[Ne] 3s² 3p³
Nature ----> Acidic
Non metal
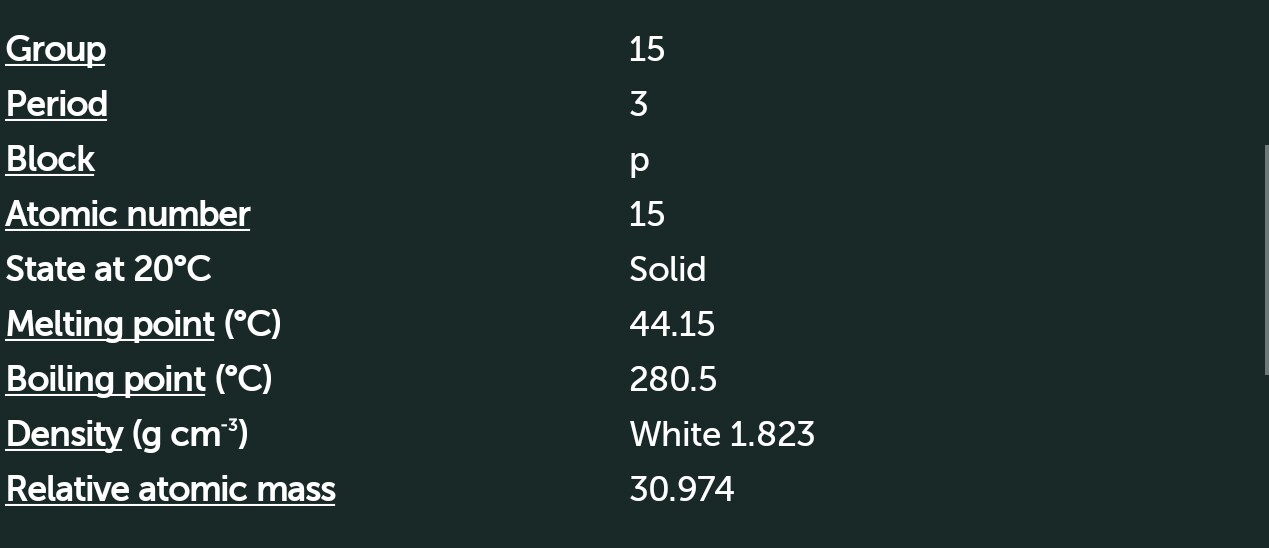
Electronegativity 2.1
Oxidation state +5,+3,-3





Arsenic

[Ar] 3d¹⁰ 4s² 4p³
Nature ----> Amphoteric
Metalloid

Electronegativity 2.0
Oxidation state +5,+3




Antimony (Sb)

[Kr]4d105s25p3
Nature ----> Amphoteric
Metalloid
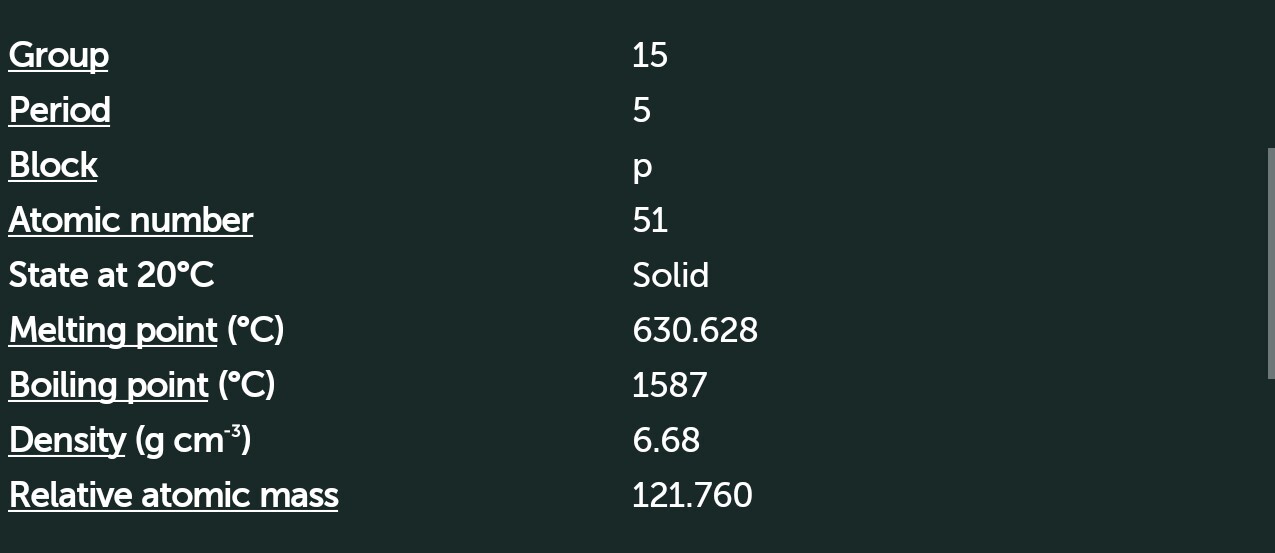
Electronegativity 1.9
Oxidation state +5,+3


Bismuth (Bi)

[Xe]4f145d106s26p3
Nature ----> Basic
Basic metal

Electronegativity 1.9
Oxidation state +3



Moscovium (Mc)

[Rn] 5f146d107s27p3
Basic metal



Note that above 92 to 118 elements are radio active elements.Thats mean Moscovium is also radio active element.
Welcome toG.A.Jr.College,Zari
Tal,Talasari
This site is made by Dipesh Gavit, 11th science.
G. A. Jr. College, Zari
If you like this blog, please share this to your friends. Thank you.
You must be logged in to post a comment.